Radiocarbon Dating
Radiocarbon Dating
GS-3: Science & Technology (UPSC/State PSC)
Context:
From thermodynamics to GPS, from social systems theory to studies of consciousness, time plays an essential role in how we study, interpret, and understand the natural universe and the peoples and technologies that occupy it. Keeping time in particular allows us to understand its passage and the change by which that passage is characterised.
- The technique called radiocarbon dating brought the first verifiable way to do this to many fields of science, transforming them- and our world-to a significant degree.
What is radiocarbon dating?
- ‘Dating’ is a method by which the age of an object can be determined. Radiocarbon dating refers to a method that does this using radiocarbon, a name for the isotope carbon-14.
Tools of radiocarbon dating:
- The Geiger counter instrument is used to study radioactive decay.
- It consists of a Geiger-Muller tube connected to some electronics that interpret and display signals.
- The Geiger-Muller tube contains a noble gas, such as helium or neon, and a rod passing through the centre. A high voltage is maintained between the tube's inner surface and the rod.
- The gas is insulating, so no current can pass between the two. During radioactive decay, gamma radiation activates electrons in the gas's atoms and produce an electric discharge.
Working Principle
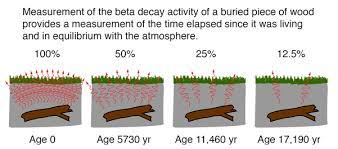
- It is based on the fact that living organisms—like trees, plants, people, and animals—absorb carbon-14 into their tissue.
- When they die, the carbon-14 starts to change into other atoms over time.
- Scientists can estimate how long the organism has been dead by counting the remaining carbon-14 atoms.
How does modern radiocarbon dating work?
- It starts with cosmic rays—subatomic particles of matter that continuously rain upon Earth from all directions.
- When cosmic rays reach Earth’s upper atmosphere, physical and chemical interactions form the radioactive isotope carbon-14.
- Living organisms absorb this carbon-14 into their tissue. Once they die, the absorption stops, and the carbon-14 begins very slowly to change into other atoms at a predictable rate.
- By measuring how much carbon-14 remains, scientists can estimate how long a particular organic object has been dead.
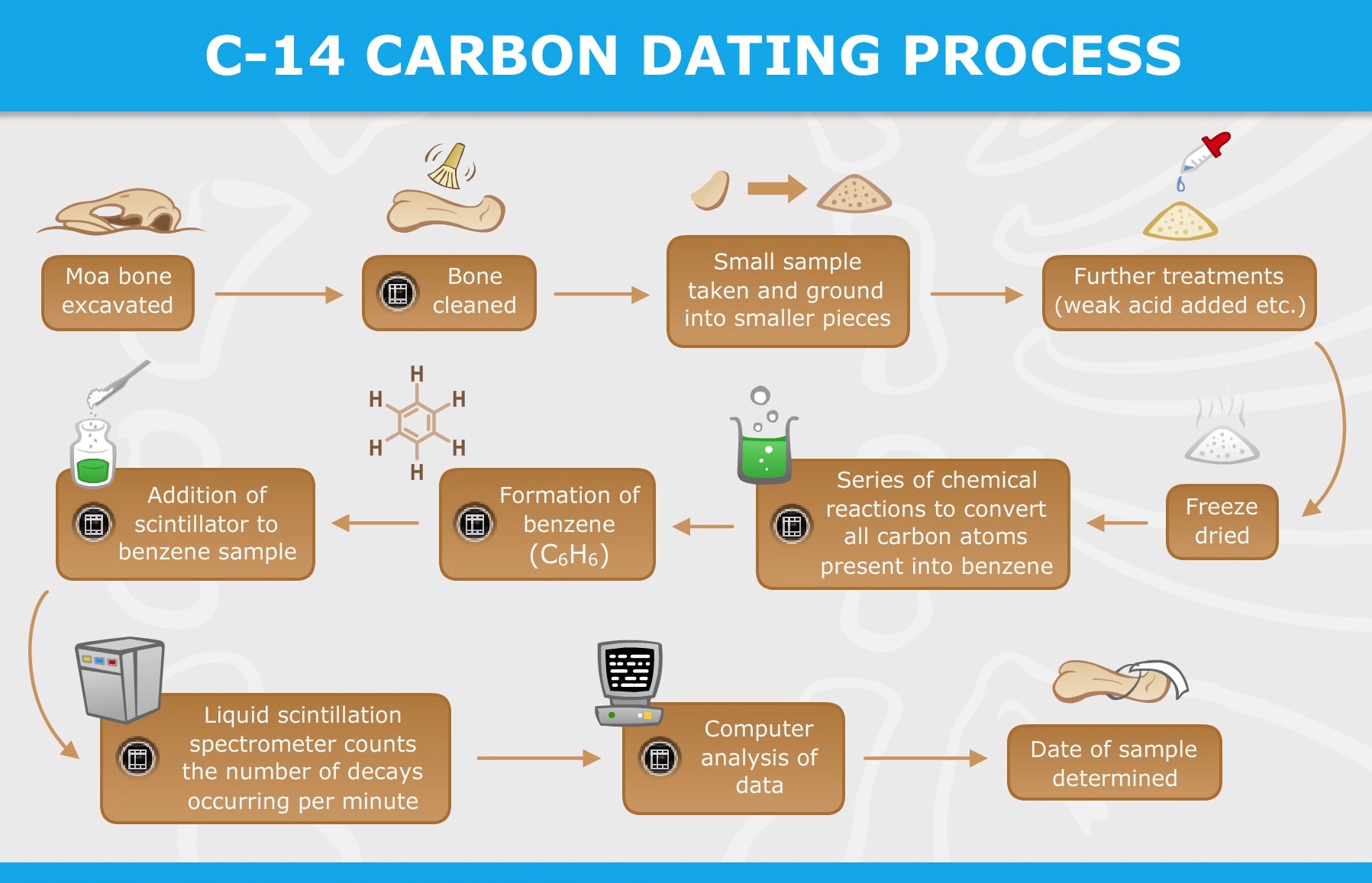
- Radiocarbon dating can be used on any object that used to be alive. That includes pieces of animals, people, and plants, but also paper that was made from reeds, leather made from animal hides, logs that were used to build houses, and so forth.
What are the limitations of carbon-14 dating?
- The various dating techniques all have limitations. Each works best for different types of problems. Radiocarbon dating works on organic materials up to about 60,000 years of age.
- Conventional radiocarbon dating requires samples of 10 to 100 grams (0.35 to 3.5 ounces) of an object, depending on the material in question. Newer forms of dating can use much smaller amounts, down to 20 to 50 milligrams or 0.0007 to 0.0018 ounces. In both cases, the material is destroyed during the test.
- Radiocarbon samples are also easily contaminated, so to provide accurate dates, they must be clean and well-preserved.
- Dirt and other matter must be washed off with water, but chemical treatments and other cleaning procedures are also often needed. This is because there are so few atoms to count; even a little extra carbon from contamination will throw off the results significantly.
- A million-year-old sample contaminated by only a tiny amount of carbon could yield an invalid age of 40,000 years, for example.
Significance of Radiocarbon Dating:
- Carbon dating has helped us reveal how our bodies work, to understand the climate of the Earth and reconstruct its history, and to track the sun’s activity and the Earth’s magnetic fields.
- Radiocarbon dating was also instrumental in the discovery of human-caused climate change, as scientists used it to track the sources of carbon in the atmosphere over time.
- The development of radiocarbon dating has had a profound impact on archaeology.
- Carbon dating not only makes it possible to compare dates of events that occurred over great distances, but it also allows for more precise dating inside archaeological sites than earlier methods.
- The consequences of carbon dating are frequently referred to as the “radiocarbon revolution” in archaeological history.
- Radiocarbon dating has allowed scientists to pinpoint the end of the last ice age and the beginning of the Neolithic and Bronze Ages in various parts of the world.
- Radiocarbon dating helped in understanding human evolution in a better way.
- In order to understand historical astronomical events and perhaps identify a trend, carbon dating is also employed to look for signs of cosmic ray activity.
Comparison with other Dating Methods
- Other dating methods have different strengths.
- Dendrochronology, also known as tree-ring dating, depends upon the preservation of certain tree species; it can extend to about 12,500 years ago for oak trees and to 8,500 years for bristlecone pine.
- Potassium-argon dating can date volcanic materials ranging from less than 100,000 to more than 4 billion years old.
- Rubidium-strontium dating can be used to determine the ages of items ranging from a few million to a few billions of years old; it is widely used to understand how the Earth and solar system formed and to trace human migration and trade in archaeology.
Radioactive Dating:
- The half-life of a radioactive element is the time it takes for half of its atoms to decay into something else. For example, the half-life of radium-226 is 1600.
- Therefore, in 1600 years, one gram of radium-226 will turn into half a gram of radium-226 and half a gram of something else
- After another 1600 years have elapsed, only a quarter of a gram of the original radium-226 will remain.
- Finding the ratio of parent to daughter elements.
Carbon Dating:
- Carbon-14 is an isotope that has a Half life of 5,700 year old.
- Half-life: The time it takes for half of the atoms in an isotope to decay.
- Radiometric Decay: Process that uses properties of atoms in rocks and other objects to determine their ages.
- Radioactive Dating: calculating the absolute age of a rock by measuring the amounts of parent and daughter materials in a rock and by knowing the half-life of the parent material
Conclusion:
In 2020, researchers from Cyprus, the Netherlands, and Russia reported a way to improve the time resolution of radiocarbon dating- the smallest period of time to which it could date objects-from decades to specific points within a year, using "recent developments in atmospheric science".
Radiocarbon dating is also of political significance in India, where researchers and politicians alike have invoked its use to date objects retrieved from temples and mosques.
Source: The Hindu
-------------------------------------
Mains Question:
What is radiocarbon dating? Throw light on the working principle, working method, limitations and significance of radiocarbon dating.